What is the secret behind the ideal gas law? Why is it that all gases behave the same (at least when this law is a good approximation to their behavior)? Also, consider a gas in a closed insulated container. If the gas is at some temperature, T, it stays at that temperature indefinitely and--more importantly--it remains a gas. Why, for instance, don't the molecules slow down and eventually fall to the bottom of the container as a liquid? Questions such as these are answered by the kinetic molecular theory of gases. As an added bonus, this model also explains why the ideal gas law is valid for all gases (provided T is not too low nor P too high).
The book has a good description of this model (theory). In essence, we find out what composes an ideal (or perfect) gas. Read the book for details; what we shall do here is look at some other aspects of what is going on. In essence, the molecules of a perfect gas can be considered to be points; that is, they have mass but, essentially, zero volume. Also, there are no interactions (attractions or repulsions) between particles. Finally, the gas molecules' only interactions are the elastic collisions with the walls of their container. These collisions cause pressure. Detailed derivations of pressure and energy can be made and are undertaken in more advanced courses. For now, it suffices to state just this:
Ideal gases are gases for which all the energy is simply kinetic energy.
The total kinetic energy of a mole of gas is just 3RT/2. In fact, the total kinetic energy of any set of particles is just this same quantity per mole! What makes gases, liquids, and solids different from each other is the addition of potential energy into the mix. In fact, real gases show the effects of potential energy. Potential energy arises whenever there are forces between the particles in question. For an ideal gas, the potential energy contribution to the total energy is zero. With real gases and with condensed phases (liquids and solids) the potential energy is important. More will be said about this in the next chapter.
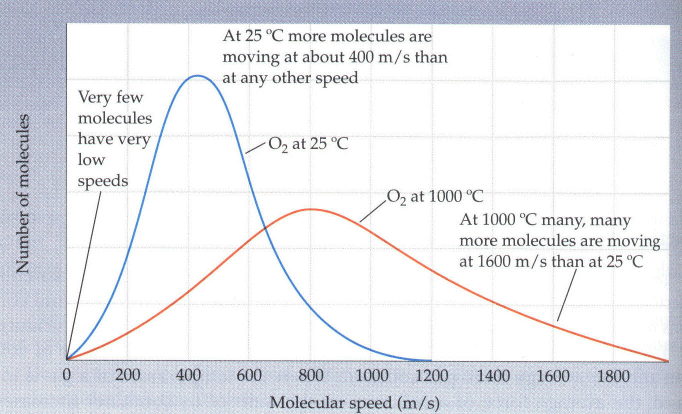
At a given temperature, the molar kinetic energy is the same for all substances. However, the potential energy can vary and this is the reason why ideal gases, real gases, liquids, and solids all have different behaviors.How do the molecules in a gas behave? The first thing we note is that the average energy per molecule remains a constant at a given temperature. However, nothing is ever quite this simple. In a given sample of gas, some molecules have greater energies and some have less. What this translates into is the fact that molecules have a distribution of speeds. This distribution changes with temperature. For instance, look at the following illustration from the book.

These curves were calculated for oxygen gas at two different temperatures. Note that the distribution of speeds approaches a bell-shaped curve at T increases. At lower temperatures, it takes on a more "skewed" appearance. We can also look at the distribution of speeds of molecules for various gases in the atmosphere. This is shown in the next picture.
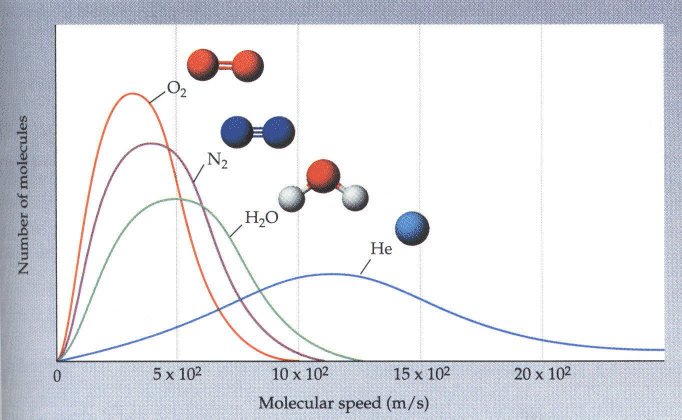
Note that heavier molecules have a more skewed distribution whereas, as molecules get lighter, the distribution looks more and more symmetrical. This is because lighter molecules move faster; remember that all have the same kinetic energy. To make up for this, lighter molecules must move much faster. This, for instance, is the reason why there is very little helium in the earth's atmosphere. The small, light helium molecules can easily attain "escape velocity" from the earth's gravitational field. This is not true--quite fortunately for us--for the heavier molecules such as water, oxygen, and nitrogen.
The book describes the relation between speed and kinetic energy. However, there is no explanation as to why there is a distribution of velocities. This distribution conforms to what is called a Boltzmann distribution of energies. This distribution can be used to find the velocities of molecules under various circumstances. In the book the symbol used for molecular velocity is u. In the equations below, I use the more commonly employed symbol, c. These equations are given without derivation. Also, only one of the speeds here is mentioned in the book. We now give some equations with minimal comments.
The first speed we look at is the rms (root-mean-square) speed or velocity. This is what is related to the kinetic energy and, if you divide the total kinetic energy of the system by the number of molecules present, you get this value. The rms velocity (speed) is just
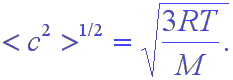
This is the formula given in the book; however, note that I have used c instead of u and that I have used a slightly different notation for averages.
The above speed is directly related to kinetic energy. Pressure is related to force, however, and not to energy. If you need to understand the force exerted by a gas on the walls of a container, you need to have the average speed of the molecules. This is given by
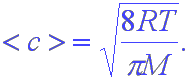
Note that this is close to the rms speed but is not equal to it. Finally, since all of the distributions you have seen have maxima, there must be a most probable speed. This is given by the formula,
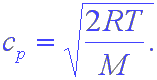
These formulas are given without derivation. Also, please note that you have to be very careful with units here. If the answers are to come out in m/s, then R must be in J/mol-K and M must be in kg/mol.
This is more than the book has given you by far. But, if you are mathematically literate, substitutions and calculations should be easily carried out. The main thing to note here is that at the same temperature, all substances have the same kinetic energy per mol.